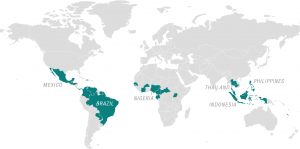
The raging pandemic of Zika virus infection occurring throughout South and Central America, the Caribbean and posing a looming threat to the United States is the most recent of four unanticipated arrivals of major arthropod-borne viral diseases in the Western Hemisphere over the last twenty years (Cook and Holmes 2006, Sternberg 2016). Zika follows dengue, which has stealthily infiltrated this hemisphere over the course of numerous decades and then aggressively emerged in the 1990s; West Nile virus, which surfaced in 1999; and chikungunya, which appeared and dominated headlines in 2013 (Chevillon et al. 2008, Cook and Holmes 2006).
Historically, Zika virus infection in adult humans has presented with mild, nonlife-threatening symptoms in twenty-percent of infected patients, with eighty-percent being clinically asymptomatic during initial infection (Faye et al. 2014). In its more than sixty years under careful observation, Zika has not been noted to induce hemorrhagic fever or death (Faye et al. 2014, Calvet et al. 2016).
Of even greater concern to the global community is the explosive epidemic of microcephaly, manifested by an apparent twenty-fold increase in incidence from 2014 to 2015, which has been linked to Zika virus infections in pregnant women (Mlakar et al. 2016, Oliveira et al. 2016, Schuler-Faccini et al. 2016). Although additional definitive evidence of a causal relationship is needed, health authorities in some afflicted regions 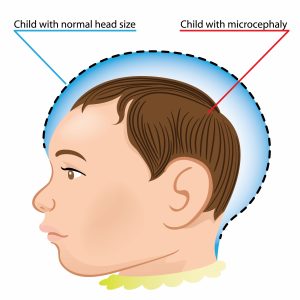 have publically recommended that pregnant women not only take strict protective precautions but also delay pregnancy altogether (Partlow 2016).
have publically recommended that pregnant women not only take strict protective precautions but also delay pregnancy altogether (Partlow 2016).
In a standalone Zika epidemic, a diagnosis could reliably be made on clinical grounds. Unfortunately, dengue and chikungunya, which have similar clinical profiles, have both been epidemic in the Americas, likely resulting in confounded clinical diagnoses of Zika virus (Chevillon et al. 2008). In such cases, when multiple arboviruses are co-circulating, viral- specific diagnoses (if available) can be critical in preventing and managing complications (Sternberg 2016).
To date, there are no Zika vaccines in advanced development, although some researchers suggest the possibility of adapting existing flavivirus vaccine platforms (Oster et al. 2016, Sternberg 2016). Zika vaccines would, however, face the same challenges as vaccines for chikungunya, West Nile, and other arboviruses (Chevillon et al. 2008, Faye et al. 2014). Since epidemics appear sporadically and unpredictably, pre-emptive vaccination of large populations in anticipation of outbreaks are often prohibitively expensive and not cost-effective. Alternatively, the stockpiling of vaccines followed by rapid deployment may not be fast enough to counter sudden explosive epidemics, like the one being experienced. Although flavivirus outbreaks have historically been prevented via offensive mosquito control, vector control has remained problematic due to complex logistics, expense, public resistance, and challenges posed by overcrowding and poor sanitation (Cook and Holmes 2006). Among the current best preventive measures against Zika virus are house screens, air conditioning and the removal of containers that store standing water and serve as mosquito breeding sites. These luxuries are often unavailable to under-resourced citizens of crowded urban areas where such epidemics hit the hardest.
The connection between Zika infection and high rates of microcephaly was initially received with skepticism (Tetro 2016). Though the likely link between Zika virus and microcephaly was initially reported in, and sourced from, French Polynesia (the likely source of the virus which preceded the outbreak in Brazil), similar reports are lacking over the many prior years that Zika has endured in its traditional endemic range (Besnard et al. 2014, Sternberg 2016, Schuler-Faccini et al. 2016, Tetro 2016).
Transmission of Zika virus can be classified as vector- and non-vector-borne (Weissenbock et al. 2010). With vector- borne transmission, several species of the genus Aedes are involved (Faye et al. 2014). The non-vector-borne transmission includes sexual transmission, perinatal or intra-uterine transmission, postnatal transmission, blood transfusion, and animal or insect bites (Faye et al. 2014, Besnard et al. 2014). Zika virus is also maintained by two transmission cycles: the sylvatic cycle, where the virus circulates between non-human primates and Aedes mosquitoes, and the urban cycle, where the 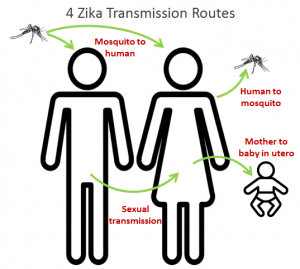 virus circulates between humans and mosquitoes (Sternberg 2016, Chevillon 2008).
virus circulates between humans and mosquitoes (Sternberg 2016, Chevillon 2008).
However, countless questions remain unanswered concerning the sexual transmission of Zika virus. Researchers remain uncertain as to how long the virus persists in the semen of infected men, whether asymptomatic men have the virus in their semen and whether Zika virus can be transmitted through oral sex (Atkinson et al. 2016).
The current incidence of Zika virus infection plaguing the Americas is difficult to gauge. This is particularly due to its nonspecific nature, its commonly mild symptomatology, the absence of uniform laboratory diagnoses available, and the flavivirus antibody cross-reactivity further complicating serologic assessment in areas in which Zika virus is endemic (Faye et al. 2014, Lanciotti et al. 2007). Nevertheless, given the historically high incidence of flaviviruses (such as dengue in the Americas), millions of Zika virus cases should be anticipated as the virus continues to spread (Chevillon et al. 2008, Cook and Holmes 2006).
If Brazil serves as an indicator for the rest of Latin America and the Caribbean, considerable numbers of infants with microcephaly and other disadvantageous pregnancy outcomes will likely surface in the forthcoming months (Sternberg 2016, Oliveira et al. 2016, Mlakar et al. 2016, Partlow 2016, Schuler-Faccini et al. 2016, Tetro 2016). The potentially significant burden of illness from Guillain–Barré syndrome, specifically, is difficult to evaluate, given the challenges with serologic diagnosis in regions where dengue is endemic and insufficient data on current incidence exists (Faye et al. 2014, Lanciotti 2007).
The underlying causes for the emergence of Zika virus in the past decade remain unknown. The recent worldwide increase in incidence and spread of dengue, chikungunya, and now Zika virus, all via Ae. aegypti, suggest shared common mechanisms responsible for their emergence (such as urbanization and globalization) (Chevillon et al. 2008). Viable explanations proposed involve viral mutations influencing virulence or transmission and viral introduction to formerly unexposed populations, resulting in an epidemic outbreak and spread (Besnard et al. 2016, Oster et al. 2016). Further research is needed to determine whether the recently associated and observed correlations between adverse birth outcomes (such as microcephaly) and Guillain–Barré syndrome reflect an increased incidence of infection, or whether they result from a shift in viral virulence (Schuler-Faccini et al. 2016, Mlakar et al. 2016, Oliveira et al. 2016). In regions of Asia and Africa where Zika virus is endemic, the incidence of infection, whether outbreaks occur, and the reason for the absence of recorded cases of adverse pregnancy outcomes or Guillain–Barré syndrome are unknown (Hayes 2009, Haddow et al. 2012, Faye et al. 2014). It is possible that exposures that occur in children, in whom Guillain–Barré syndrome is unlikely to develop, would later be immune to such infections during pregnancy (Hayes 2009, Haddow et al. 2012, Faye et al. 2014).
It is safe to state that the long-term trajectory of the ongoing Zika virus outbreak in the Americas is daunting and uncertain. The development and use of herd immunity to slow and control further transmission of the virus could occur, although this should not hinder the urgent need for immediate and long-term prevention and implementation of effective control strategies (Faye et al. 2014, Besnard et al. 2016, Oster et al. 2016, Sternberg 2016). Whether and where Zika virus becomes endemic (in the future) or an enzootic transmission cycle develops in the Americas are merely matters of inference (Oster et al. 2016). Critical thinking and developed hypotheses are important for the long-term development and sustainability of countermeasures.
Our team devised a three-step method (including collecting research and information; conducting surveys and analyzing data; and utilizing ethnographic interviewing techniques to explore areas of relevant interest further) that will ideally allow us to be as productive as possible once we are in-country. To avoid the arrogance of assuming that we come knowing the best implementation and teaching practices, we will use the described surveys to measure what is and is not known, and gain insight as to how to best deliver such information.
This method is highly mobile and adaptable and promotes patient autonomy. This format will best ensure that our efforts result in a respectful and productive dialogue. Furthermore, the surveys enable patients to ask questions of us not directly related to our topic, which compliments the Uncertainty Reduction Theory and inspires mutual trust and conversation.
The embedded presentation specifically addresses Zika Virus and Maternal Health and synthesizes the above information in a Nicaragua [local]-centric manner and provides additional support in the forms of infographic handouts, educational flyers and culturally and contextually appropriate surveys that will be used during our time in-country.
(For reference, see Research).